AI Stock Showdown: 6 Chatbots Forecast Novo Nordisk After FDA Win

Disclaimer: This article is for informational and educational purposes only. The predictions and analyses presented herein were generated by AI systems and should not be construed as financial advice, investment recommendations, or solicitations to buy or sell any securities. Stock prices are inherently unpredictable, and all investments carry risk of loss. Past performance does not guarantee future results. Readers should consult qualified financial advisors before making any investment decisions. TheDayAfterAI News and its contributors do not accept liability for any losses arising from reliance on this content.
In this analysis, TheDayAfterAI News tasked six leading AI chatbots — ChatGPT, Claude, Gemini, Grok, Copilot, and Perplexity — with predicting Novo Nordisk's (NVO) stock performance for the trading week of December 23–29, 2025. This comparison comes at a pivotal moment: the FDA approved Novo Nordisk's oral Wegovy pill on December 22, 2025, marking the first-ever oral GLP-1 medication for obesity in the United States.
All six AI platforms reached a bullish consensus, though their probability assessments and price targets varied significantly. This article presents our methodology, comparative findings, and key insights for investors navigating AI-assisted market analysis.
The Catalyst: FDA Approval of Oral Wegovy
On December 22, 2025, the U.S. Food and Drug Administration approved Novo Nordisk's oral semaglutide (Wegovy pill) for weight management in adults with obesity or overweight. Key details include:
First oral GLP-1 medication approved for obesity in the United States
Clinical trials showed 16.6% mean weight loss with treatment adherence
Launch expected in early January 2026 at $149/month
Copenhagen trading showed approximately 7–10% gains following the announcement
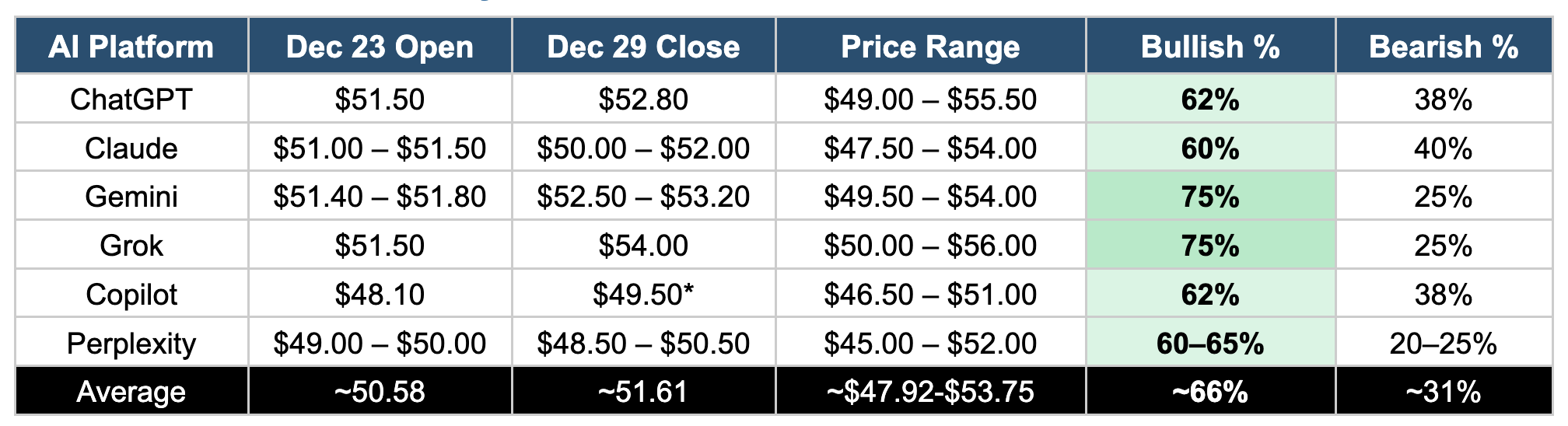
Comparative Analysis: Head-to-Head Results
Gemini – Most Bullish Outlook
Gemini presented the most optimistic scenario with a 75% probability of upside. The analysis emphasized the FDA approval as a decisive catalyst that would allow Novo Nordisk to bypass injection pen manufacturing bottlenecks, significantly expanding the total addressable market. Gemini predicted the stock would break out of its recent consolidation phase ($43–$50), with the previous resistance ceiling of $50 becoming critical support.
Key Insight: Gemini highlighted a potential "short squeeze" scenario given the high put/call ratio prior to the news.
Grok – Highest Price Target
Grok projected the highest Dec 29 closing price at $54.00, representing an 12.3% gain from the previous close. The analysis noted the falling wedge pattern on daily charts suggesting a potential reversal, with the FDA approval acting as a breakout trigger. Grok identified resistance at $55 (prior highs) and potential extension to $60 if momentum builds.
Key Insight: Grok emphasized unusual call volume for December 26 options expiration, signaling bullish institutional bets.
ChatGPT – Balanced Scenario Analysis
ChatGPT delivered a structured scenario-based analysis with 62% probability of upside. The platform provided detailed day-by-day estimates and identified a $50–$50.50 gap support zone as the key level to watch. ChatGPT noted the neutral-to-slightly-bearish pre-catalyst technicals (RSI ~47–48, negative MACD) but acknowledged the overwhelming positive catalyst.
Key Insight: ChatGPT flagged that if NVO loses the $50 support area, "sell-the-news" risk rises quickly.
Claude – Most Comprehensive Technical Analysis
Claude provided the most detailed technical framework with 60% probability of gains, offering a more cautious "Cautiously Bullish" stance. The analysis included scenario probabilities: Bullish (45%, $52–$56), Base Case (40%, $48–$52), and Bearish (15%, $45–$48). Claude emphasized that much of the good news may be priced in quickly given NVO's 41% YTD decline.
Key Insight: Claude noted upcoming competition from Eli Lilly's orforglipron as a medium-term headwind.
Perplexity – Most Extensively Sourced
Perplexity provided 60–65% probability of gains with the most comprehensive citation framework (58 sources). The analysis offered nuanced day-by-day scenarios accounting for options expiration pinning effects on December 26. Perplexity's wider range ($45–$52) reflected acknowledgment of holiday liquidity risks and structural uncertainties.
Key Insight: Perplexity uniquely highlighted options dealer hedging dynamics that could pin prices near major strikes during the December 26 expiration.
Copilot – Pre-Catalyst Baseline
Copilot's analysis did not incorporate the FDA approval news, providing an interesting counterfactual baseline. Without the catalyst, Copilot projected modest gains of –1% to +3%, demonstrating how fundamentally the FDA approval shifted the outlook for all other platforms.
Key Insight: Copilot's pre-catalyst analysis serves as a control case, showing the approximate 5–7% uplift the FDA news contributed to price targets.
Key Findings
Universal Bullish Consensus: All six platforms predicted a positive week for NVO, with bullish probabilities ranging from 60% to 75%. No platform assigned greater than 40% probability to a bearish outcome.
Gap-Up Convergence: Five of six platforms (excluding Copilot's pre-catalyst analysis) predicted a significant gap-up opening on December 23, with estimates clustering around $51–$52.
Holiday Liquidity Risk: All platforms flagged the holiday-shortened trading week as a source of amplified volatility, with thin liquidity potentially exaggerating price movements in both directions.
Support Level Alignment: Multiple platforms identified $50 as the critical support level, with a break below potentially triggering "sell-the-news" pressure.
Divergent Confidence Levels: Gemini and Grok showed highest conviction (75% bullish), while Claude and Perplexity expressed more measured optimism (60–65%), reflecting different risk assessment frameworks.
Conclusion
The AI chatbot consensus on NVO reflects the market's positive reception to a transformative regulatory milestone. While probability assessments varied from 60% to 75% bullish, all platforms recognized the FDA approval as a genuine game-changer for Novo Nordisk's competitive positioning in the obesity treatment market.
For investors, this comparison demonstrates both the utility and limitations of AI-assisted analysis. The convergence on directional outlook provides a useful sentiment check, while the variance in specific price targets reminds us that even sophisticated AI models handle uncertainty differently.
TheDayAfterAI News will follow up with an accuracy assessment after December 29, 2025, comparing each platform's predictions against actual market outcomes. Stay tuned for our post-analysis report.
License This Article
We are a leading AI-focused digital news platform, combining AI-generated reporting with human editorial oversight. By aggregating and synthesizing the latest developments in AI — spanning innovation, technology, ethics, policy and business — we deliver timely, accurate and thought-provoking content.